Drug Discovery
Therapeutic large molecular drugs are an important class of medicines serving patients most in need of novel therapies. This class of drugs including peptide, protein and antibody drugs. Among these drugs, antibody related therapies contributed the major percentage of investments and clinical medicines.
Antibody Drug Discovery
Therapeutic antibodies are monoclonal antibodies developed to attack specific cells or proteins for the treatment of a wide variety of diseases, including immune disorders, cancers, and infections. The anti-tumor effect is very well studied and the most widely used among all of the antibody therapy applications.
Advantages of Modern Therapeutic Antibodies
Advantages of Modern Therapeutic Antibodies
- High-affinity
- High specificity and low off-target activity
- Low or non immunogenic
- Long-term benefit with short-term therapy
- Endotoxin free
|
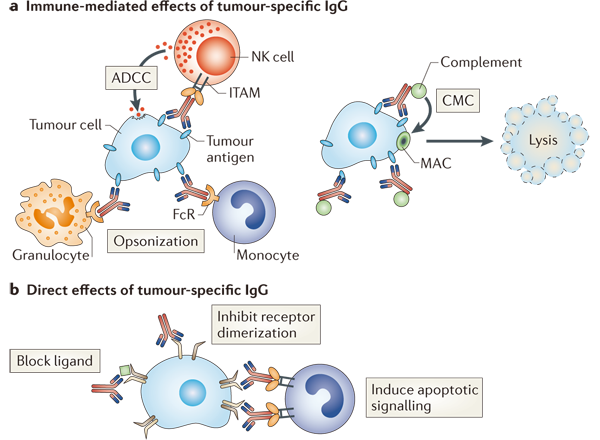
Machenism of ADCK (Antibody Directed Cell Killing) [1]
Monoclonal antibodies (mAbs) that bind directly to cancer cells can direct their antitumour effects through various mechanisms. These mechanisms are routinely identified in vitro. a | Antibody can mediate antibody-dependent cellular cytotoxicity (ADCC) by immune effector cells that express immunoreceptor tyrosine-based activation motifs (ITAMs), such as natural killer (NK) cells, monocytes, macrophages and granulocytes. Fixation of complement can induce the opsonization of the target cell and thereby enhance phagocytosis and lysis by monocytes and granulocytes. Complement-mediated cytotoxicity (CMC) can directly result in cell death through the development of a membrane attack complex (MAC). b | mAbs can also have direct effects on target cells by blocking the binding of an activating ligand that is responsible for the survival of the cancer cell, inhibiting the dimerization of a receptor, thereby blocking an activation signal or inducing an apoptotic signal by crosslinking a receptor. Such crosslinking of the receptor can be enhanced when a mAb is bound to Fc receptor-expressing cells. |
|
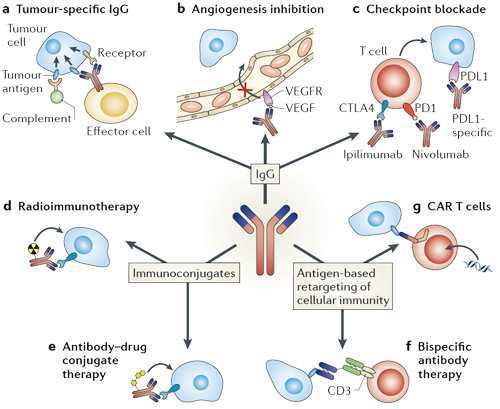
Machenism of ABCK (Antibody-Based Cell Killing) [1]
Current monoclonal antibody (mAb) therapeutics are set based on the following strategies. Antibody that bind to target cancer cells (a) can mediate antibody-dependent cellular cytotoxicity (ADCC) by immune effector cells, induces complement-mediated cytotoxicity (CMC) or lead to the direct signalling-induced death of cancer. IgG antibodies can also be used to inhibit angiogenesis (b) or to block inhibitory signals (c), thereby resulting in a stronger T cell antitumour response. Radioimmunoconjugates (d) deliver radioisotopes to the cancer cells, whereas antibody–drug conjugates (e) carry highly potent toxic drugs to the cancer cells. mAb variable regions are also used to retarget immune effector cells towards cancer cells through the use of bispecific mAbs that recognize cancer cells with one arm and activating antigens on immune effector cells with the other arm (f) or through a gene therapy approach in which DNA for a mAb variable region fused to signalling peptides is transferred to T cells, thereby rendering them chimeric antigen receptor (CAR) T cells (g) specific for the tumour. CD3: T cell surface glycoprotein CD3 ε-chain; CTLA4: cytotoxic T lymphocyte-associated antigen 4; PD1: programmed cell death protein 1; PDL1: PD1 ligand; VEGF: vascular endothelial growth factor; VEGFR: VEGF receptor. |
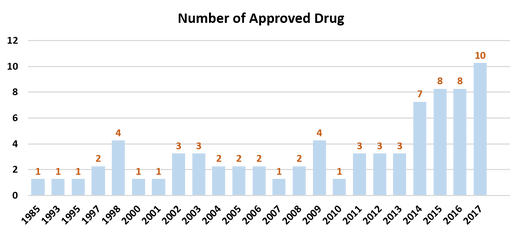
The number of approved and under-investigated antibody drug keeps on increasing on its fast pace. In the period of 2011 to 2016, 62 protein drugs are approved by FDA and 30 of which are antibody drugs[2]. In the 2018 FDA novel drug approval list, 12 out of 59 are antibody drugs including the superstar PD-1 inhibitor Cemiplimab[3]. Driven by the development in biomarker discovery and antibody operation technology, more and more first-in-class therapeutic antibodies are set to join in the clinical trial phase. Further more, under the patent law a large amount of bio-similar will enter the market with increased competition.
No matter you are rising a novel antibody drug or bio-similar, to maximize the success and minimize the risk are essential to thoroughly examine every aspect of the therapeutic antibody drug discovery process. What is more important: it is not only about technology, but also about the whole project management and key point control. Bio Bench senior team composes of talent researchers and project managers from leading scientific and commercial groups. Till now we have operated more than 30 antibody drug projects and 2 of them are already in clinical trial phase.
Procedure |
Description |
|
|
|
|
Binder discovery |
|
Lead antibody discovery |
|
Antibody engineering: humanization and affinity maturation |
|
|
|
Druggability test |
|
In vivo investigation |
|
[1] George J. Weiner. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer (2015). Jun;15(6):361-70. source
[2] H.A. Daniel Lagassé, Aikaterini Alexaki, Vijaya L. Simhadri, et al. Recent advances in (therapeutic protein) drug development. F1000Res (2017). Feb 7;6:113. source
[3] Asher Mullard. Nature Reviews Drug Discovery (2019). 18, 85-89. source
[2] H.A. Daniel Lagassé, Aikaterini Alexaki, Vijaya L. Simhadri, et al. Recent advances in (therapeutic protein) drug development. F1000Res (2017). Feb 7;6:113. source
[3] Asher Mullard. Nature Reviews Drug Discovery (2019). 18, 85-89. source
|
Brochure for drug discovery? Click HERE to start downloading!
|
|