Mammalian-ST™ Stable Cell Line / Stable Pool Development Service
|
Service and Timeline |
Service Description (To be determined according to your specific
project) |
Deliverables |
Price |
|
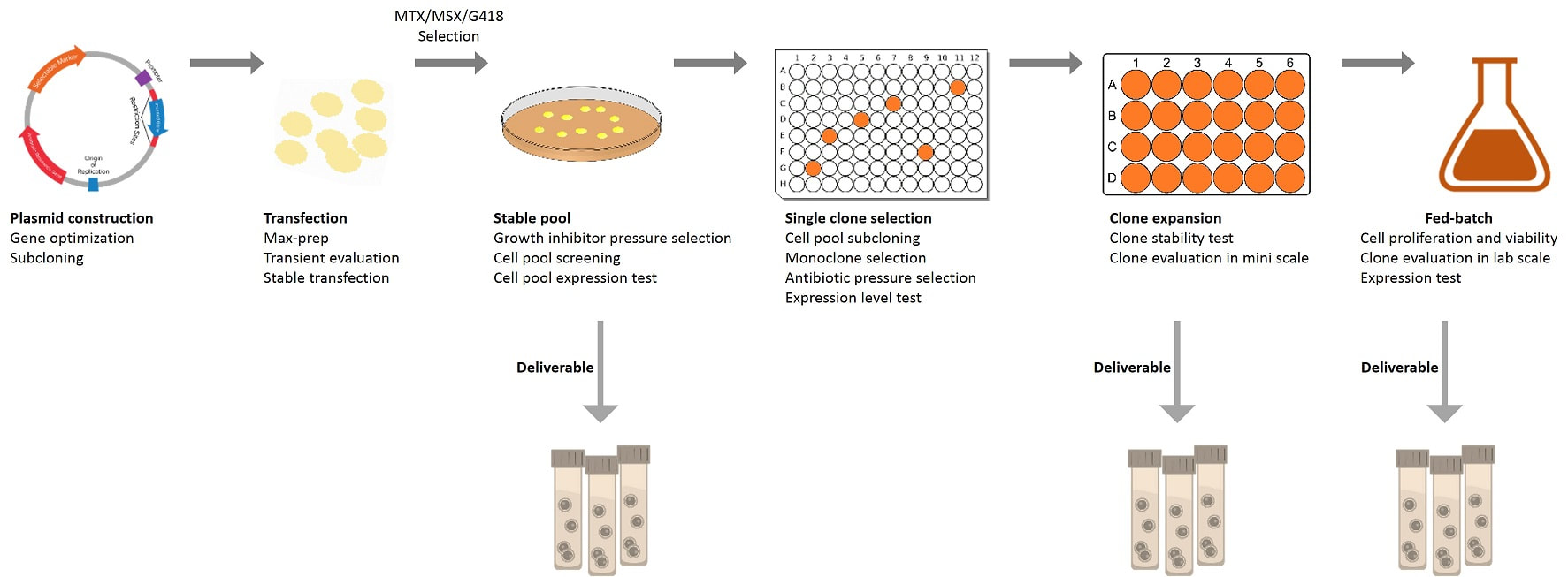
P1521 – Stable pool Mammalian stable pool generation
service 8 – 10 Weeks |
1. Positive control
protein/antibody characterization. 2. Codon optimization, gene
synthesis, cloning and plasmid preparation. 3. Transient transfection test and
antibiotic concentration determination. 4. Transfection and stable pool
generation. 5. Stable pool characterization:
expression level, cell viability, growth curve, etc. More characteristics
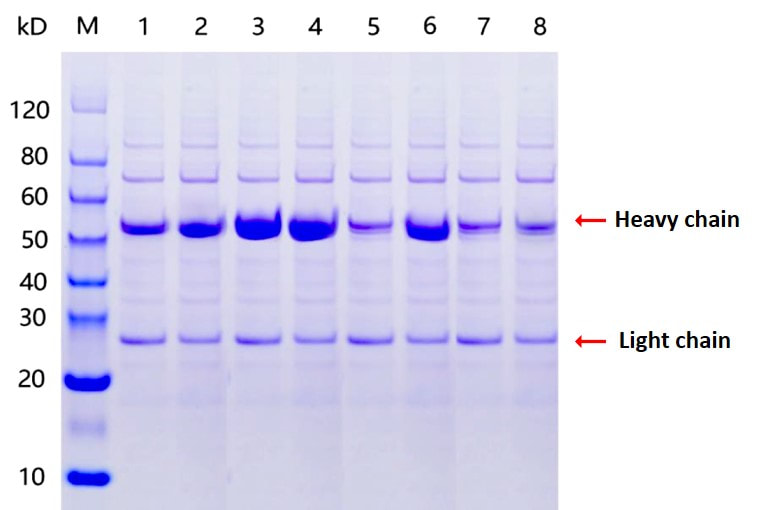
optional! 6. SDS-PAGE, WB, ELISA, and/or FACS
analysis. 7. Protein sample expression and
purification. 8. QC and report. |
- Selected and optimized stable
pool. - Purified protein sample. - Data and QC report. |
|
|
P1526 – Stable cell line Mammalian stable cell line
generation service 23 – 30 Weeks |
All of the content from P1521
Stable pool generation service, together with the following: 1. Monoclonalization
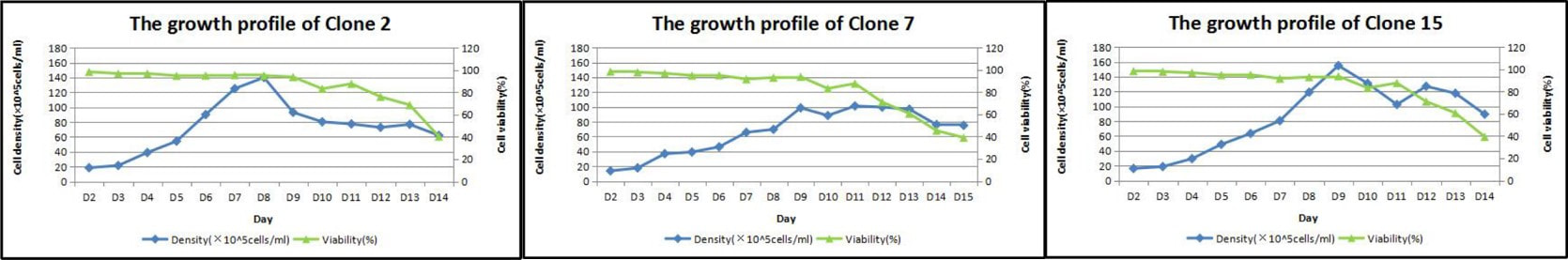
and best clones selection. 2. Fed-batch 1 (small culture
volume) primary study and characterization. At least 6 characteristics will
be tested for a duration of 8 – 15 days. 3. Fed-batch 2 (midi culture
volume) enhanced study and characterization, optional. 4. Cell banking. 5. Whole genome sequencing of the
selected clone, and backup plasmid establishment. |
- Selected stable cell line. - Purified protein sample. - Data and QC report. |
|
|
More
test options, including: 1. Bioactivity test: binding/blocking
assay, ADCC assay, etc. 2. Affinity test by biacore or ForteBio method. 3. Cell culture monitoring: Ammonia
test, lactic acid test, pCO2 analysis, etc. 4. Charge heterogeneity test. 5. MS test: molecular weight,
aggregation, etc. Would like to lean more? Seeking a
specific testing method? Click on Inquiry and we will get back to you soon. |
|||
|
Bio Bench has successfully established the following
host into stable cell line |
|||
|
CHO |
CHO-K1 |
expiCHO |
CHO-S |
|
293F |
CHO-DG44 |
HEK293 |
293T |
|
expi293F |
Vero (insect cell) |
Your cell line here! Get
a quote for free |
|
Several typical applications including:
- Hit screening / identification in therapeutic drug discovery.
- Cell signaling.
- Protein production, including antibody drug production.
- Toxicity study.
- Gene/mRNA function study.
General process of stable cell line development
Transient expression and its pros and cons
Stable pool/Stable cell line and its pros and cons
Comparison among transient expression, stable pool and stable cell line
|
Service type Specification |
Transient transfection |
Stable pool |
Stable cell line |
|
Initial cost |
+ |
++ |
+++ |
|
Midi scale cell culture or production cost |
+++ |
++ |
++ |
|
Large scale cell culture or production cost |
+++++ |
++ |
+ |
|
Operation complexity at small scale |
++ |
+ |
+ |
|
Operation
complexity at midi scale |
+++ |
+ |
+ |
|
Operation complexity at large scale |
+++++ |
+ |
+ |
|
Operator-to-operator variation |
++++ |
+ |
+ |
|
Batch-to-batch variation (by the same operator) |
++ |
+ |
+ |
|
Protein expression level |
+ |
+++ |
+++++ |
|
Short term expression stability (1 - 3 days) |
++++ |
+++++ |
+++++ |
|
Long term
expression stability (10 – 20 days) |
\ |
++ |
+++++ |
|
Regeneration stability |
\ |
++ |
+++++ |
|
Conclusion |
1. Cell culture volume <100mL, or occasionally used cell culture. 2. Protein amount <10mg, or occasionally used protein. 3. Transfection is request by experiment protocol. 4. Co-transfection of more than 1 plasmid. 5. Small scale and
proof-of-concept experiment. |
1. 100mL < Cell culture volume < 20L, or regularly used cell
culture (without specific requirement). 2. 10mg < Protein amount < 5g, or one time production but enough
for a long period of usage. 3. Non-commercial purpose, or less sensitive to cost. 4. Low/no requirement for cell homogeneity. 5. Low requirement
for stability. |
1. Small to high volume cell culture, with or
without high requirement. 2. Small to high amount of protein production. 3. Commercial purpose, cost effective. 4. Experiment requires high level of cell
homogeneity. 5. High requirement for cell stability. |
|
|
Case study: Mammalian-ST™ Stable pool/Stable cell line
|
A drug potential antibody was discovered from human naive antibody library. After in-house test, one of the selected clones was considered “great performance”, and further experiment will require large amount of purified antibody. Client requests Bio Bench to establish a stable cell line and purify optimum quantity of antibody.
CHO-K1 was selected as expression host to establish the stable cell line. G418 was chosen as selection marker in order to simplify the process. A vector containing G418 resistance was use to carry the antibody gene. After the operation, 1.2g/L titer was achieved by Bio Bench. Would like to learn more? Request full case study! |
|